D Glyceraldehyde Fischer Projection
Depending on the number of carbon atom they are further classified into trioses tetroses pentoses hexoses etc which is further. It is an aldohexose and a C-4 epimer of glucose.

File D Glyceraldehyde 2d Fischer Svg Wikipedia
It is the carbon derived from the carbonyl of the ketone eg.

. Mild traumatic brain injury mTBI is the most common type of traumatic brain injury and it leads to temporary. In assigning the D and L configurations of sugars we could direcly look for the OH group of the bottom asymmetric carbon in the Fischer projection. That is it cannot be superimposed onto it.
Sur lillustration la formule semi-développée de dessus correspond à une Projection de Fischer alors que celle de dessous est une représentation de Cram. Life Sciences have always been a fundamental area of science. A galactose molecule linked with a glucose molecule forms a lactose molecule.
Nicholas Port 1 Dr. See DL convention Step 2. Les aldoses sont lune des deux familles doses avec les cétoses.
C-5 is the anomeric reference atom. In the 1950s James D. Like most carbohydrates simple aldoses have the general chemical formula C n H 2 O n.
Việc hoán đổi vị trí D hay L được thực hiện dựa trên hướng của cárbon bất đối xứng xa nhất tính từ nhóm carbonyl. Recent work suggests that SHH MB arises from a rare population of MB-propagating cells MPC expressing the transcription factor SRY sex determining region Ybox 2 SOX2 18 19These cells actively divide during tumor initiation and hierarchically contribute to the hyperplasia of the GCPs before acquiring a stem-like quiescent transcriptional profile in full. Soit une fonction cétone en position 2 principalement 3 ou 4 cétopentoses.
Mira Antonopoulos 1 Dr. PDF On Jan 1 2008 Dr. La fonction carbonyle est une fonction aldéhyde et elle définit la position du premier carbone.
In 1 C-1 is behind the plane of the paper and the hydroxy group on C-5 is in front. Andrea Hohmann 1 Dr. It is the 2-D structure of the molecule.
Trong hệ tọa độ Fischer chuẩn nếu nhóm hydroxyl nằm ở bên phải thí. Number the carbon chain in 1 starting at the top. Si au contraire le groupe carbonyle est situé sur une autre position lose est une cétone on parle.
Les pentoses sont des oses monosaccharides qui comportent 5 atomes de carboneIls ont tous la même formule brute C 5 H 10 O 5. 1 Indiana University Bloomington IN United States. The following is a definition of D and L notations.
Because formaldehyde n1 and glycolaldehyde n2 are not generally considered to be carbohydrates the simplest possible aldose is the triose glyceraldehyde which only contains three carbon atoms. Les aldopentoses ont 3 centres de. Of these D-isomers all except D-altrose occur in living organisms but only three are common.
The easiest way of showing the atomic arrangement of the monosaccharide is the Fischer projection of the molecule. Academiaedu is a platform for academics to share research papers. This refers to their affinity for a specific isomer of glyceraldehyde.
In aldohexoses the anomeric reference atom is the stereocenter that is farthest from anomeric carbon in the ring. La plupart des fruits contiennent du D-fructose soit sous forme libre soit sous forme de résidu dans le saccharose sucre de table 10 rapidement hydrolysé dans lintestin en D-fructose et D-glucoseDe très nombreux produits alimentaires contiennent du D-fructose à travers lutilisation en tant quingrédients du sucre et du sirop de maïs à haute teneur en D-fructose HFCS. To generate the pyranose ring the oxygen atom on C-5 in 1 needs to be attached to C-1 by a single bond.
Monosaccharide đơn giản nhất có n 3 là các dihydroxyacetone và D- và L-glyceraldehyde. Si le groupe carbonyle est situé à une des extrémités de la chaîne carbonée lose est un aldéhyde on parle daldose. Fischer projection of D-glyceraldehyde.
However L-altrose has been isolated from strains of the bacterium Butyrivibrio fibrisolvens. Le d-ribose a ces groupes hydroxyle sur le côté droit et est associé au nom systématique 2R 3R 4R -2345-tétrahydroxypentanal tandis que le l-ribose a ses. In 1958 George Beadle and Edward Tatum received the Nobel Prize for work in fungi showing that one gene produces one enzyme.
It was at some time decided that -glyceraldehyde was the D-enantiomercitation needed The configuration of other chiral compounds was then related to that of -glyceraldehyde by sequences of chemical reactionsFor example oxidation of -glyceraldehyde 1 with. D-glucose D-galactose and D-mannoseThe L-isomers are generally absent in living organisms. Draw the Fischer projection of the acyclic form of D-glucose.
Galactan is a polymeric form of galactose found in. They are D-isomer and L-isomer. Gabriel Nah 1 Ms.
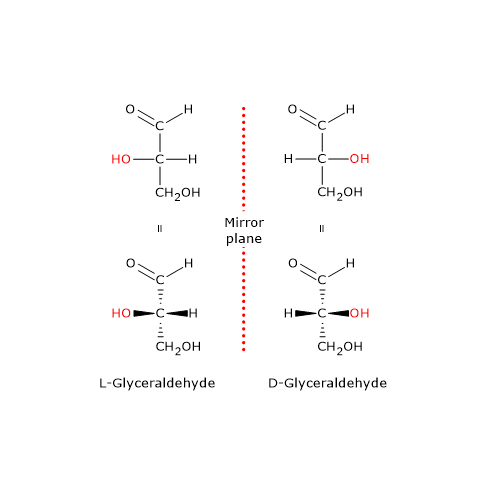
Glyceraldehyde has one asymmetric carbon atom and comes in two enantiomeric forms as shown. To illustrate using present day knowledge Fischer projection formulas and names for the D-aldose family three to six-carbon atoms are shown below with the asymmetric carbon atoms chiral centers colored red. Monosaccharide nomenclature is the naming system of the building blocks of carbohydrates the monosaccharides which may be monomers or part of a larger polymerMonosaccharides are subunits that cannot be further hydrolysed in to simpler units.
The last chiral center in an aldose chain farthest from the aldehyde group was chosen by Fischer as the D L designator site. The word chirality is derived from the Greek χειρ kheir hand a familiar chiral object. Suman Khowala and others published Carbohydrates Find read and cite all the research you need on ResearchGate.
Il existe 2 isomères de lérythrose le L-érythrose et celui qui est prépondérant dans la nature le D-érythrose. The letters D or L before the name of any compound indicate the stereoisomers relative configuration. The position of the OH group of the penultimate carbon determines whether a monosaccharide is a D-isomer or an L-isomer.
In 1988 Colin. Soit une fonction aldéhyde en position 1 aldopentoses. If its located on the right we designate it with D and vice versa since they would have the same relative configurations with glyceraldehyde for the bottom asymmetric carbon.
The exponential increase in the quantity of scientific information and the rate at which new discoveries are made require very elaborate interdisciplinary and up-to-date information and. Dans sa forme linéaire le ribose peut être reconnu comme le sucre pentose dont tous les groupes fonctionnels hydroxyle sont du même côté dans sa projection de Fischer. We would like to show you a description here but the site wont allow us.
Galactose ɡ ə ˈ l æ k t oʊ s galacto- -ose milk sugar sometimes abbreviated Gal is a monosaccharide sugar that is about as sweet as glucose and about 65 as sweet as sucrose. 1 Fischer projection with C-1 at the top the anomeric centre. Different projections of α-D-glucopyranose.
Conversely a mirror image of an achiral object such as a sphere. DBA03 EPISODIC MEMORY IMPAIRMENT FOLLOWING MILD TRAUMATIC BRAIN INJURY. Until 1951 it was not possible to obtain the absolute configuration of chiral compounds.
Chirality k aɪ ˈ r æ l ɪ t iː is a property of asymmetry important in several branches of science. An object or a system is chiral if it is distinguishable from its mirror image. Ils possèdent tous un groupement carbonyle.
Watson Francis Crick Rosalind Franklin and Maurice Wilkins were instrumental in solving DNA structure and suggesting its relationship with genetic transfer of information. When drawn in this order the Fischer projections of the D-aldohexoses can be identified with the 3.
The Fischer Projection Chemgapedia
Fischer Rosanoff Convention Or D L System Tuscany Diet
The Fischer Projection Chemgapedia

Glyceraldehyde An Overview Sciencedirect Topics
0 Response to "D Glyceraldehyde Fischer Projection"
Post a Comment